Analysis of main problems and causes of clinical use of in vitro diagnostic reagents in China
Release time:
2019-12-06
I. types of in vitro diagnostic reagents actively used in medical institutions
At present, about 75% of the reagents used in the laboratory department of large tertiary hospitals in China are imported products, especially the luminescent tumor markers, sex hormones, thyroid hormones, blood coagulation, blood cells and other reagents are almost all imported. At present only biochemical reagent domestic ratio is relatively high. Biochemical reagents in primary and secondary hospitals are almost all domestic, and tertiary hospitals also have a high proportion of domestic reagents. Enzyme-labeled infectious reagents (such as preoperative eight items) were all domestic reagents.
About 1000 in vitro diagnostic reagents are actively used in large medical institutions. There are about 500 kinds of in vitro diagnostic reagents routinely used in the laboratory department of the tertiary comprehensive hospital, 250~300 kinds in the pathology department, 250 kinds of auxiliary reagents for flow cytometry, and dozens of special reagents in each department. Some extra-large hospitals, such as a hospital, use nearly 2000 kinds of in vitro diagnostic reagents, the hospital has more than 600 kinds of laboratory, more than 620 kinds of pathology, more than 480 kinds of blood diseases laboratory, more than 170 kinds of various specialized laboratories.
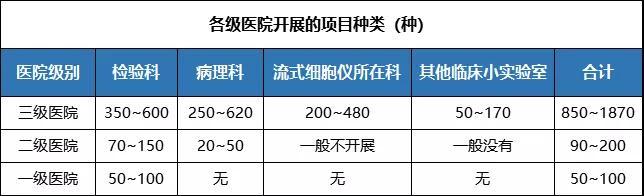
Secondary hospitals generally carry out biochemistry, two halves, three routine, coagulation and other dozens of testing items. Level 1 hospitals carry out a very limited number of tests, usually biochemical and three routine tests.
Ii. In vitro diagnostic reagent import and domestic situatio
At present, about 75% of the in vitro reagents used in the laboratory department of large-scale tertiary hospitals in China are imported products, especially the luminous tumor markers, sex hormones, thyroid hormones, blood coagulation, blood cells and other reagents are almost all imported. At present only biochemical reagent domestic ratio is relatively high. Biochemical reagents in primary and secondary hospitals are almost all domestic, and tertiary hospitals also have a high proportion of domestic reagents. Enzyme-labeled infectious reagents (such as preoperative eight items) were all domestic reagents.
There are two main reasons for the high proportion of imported reagent in large hospitals:
Large inspection equipment to import - based, domestic equipment has not become the mainstream. With the exception of biochemical tests, luminescent instruments are all "closed systems" that use bar codes to lock reagents, making them incompatible with other companies' reagents. In addition, some industry experts promoted the concept of "better quality of proprietary closed reagent", which helped import manufacturers to control the supply of consumables by occupying the space of instruments. In addition, large hospitals have high requirements for reagent instruments and believe that the quality of domestic reagents is not as good as imported reagents, so most large hospitals choose imported reagents. Domestic reagent and instrument research and development level and product quality also need more expert trust and time to accumulate.
Iii. Procurement and logistics of in vitro diagnostic reagent
Many first-class hospitals are purchased by the government procurement platform, which has a single procurement channel and simple management. They only purchase products with registration certificates. Secondary hospitals, part of the government procurement platform unified procurement and supply, part of the independent procurement.
There are 1000~2000 kinds of large tertiary hospitals, with many suppliers. Some hospitals have adopted electronic software systems for purchasing and supplier management. Supply information is more complete, management is more orderly. However, the archiving management of registration certificate in many hospitals is worrying, and the registration certificate is expired or incomplete.
At present, no matter the tertiary hospitals or the primary or secondary hospitals, they do not pay enough attention to the cold chain distribution of diagnostic reagent. If use simple plastic bags to take the subway delivery, no ice and basic foam box protection measures. The reasons for neglecting cold-chain transport are also varied. The inspectors said "experience has shown" that short city trips do not have cold chains and do not affect reagent quality. In addition, the use of in vitro diagnostic reagents ordered by the hospital is high frequency, the single goods are few, the cold chain transport is not practical, this is also a common reflection of the problem. In the hospital to receive this link, most of the procurement department personnel only pay attention to the type and quantity of goods, a simple look at the appearance of the received, for whether there is cold chain transport, generally do not care.
DE sheng is a professional in vitro diagnostic reagent research and development manufacturers, is professional in the development, production, sales of in vitro diagnostic reagent institutions, companies focus on the in vitro diagnostic reagent industry. Since its establishment, the company has always abided by and adhered to the core business philosophy of "taking morality as the foundation and honesty as the first", and made it our duty to provide value to customers. At present, it has established cooperative relations with 400 domestic and foreign enterprises.
Previous page
Next page
Previous page
Next page
Contact details
Contact number
Address: C8, Guanggu United Science and Technology City, Ezhou City, Hubei Province
Fax:0711-3704 589
Follow us



